- Uncontrolled proliferation of hematopoietic precursor cells with loss of maturation and differentiation
- The malignant cells (blasts) take over the BM and suppress normal haematopoiesis
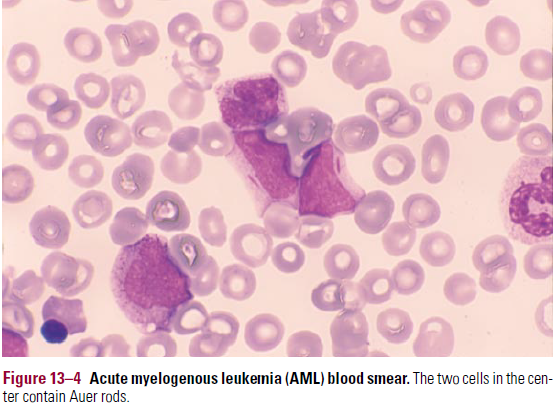
- Auer rods – is characteristic of AML
- Linear reddish cytoplasmic inclusions, diagnostic of myeloid lineage
Complications
- Suppression of normal hematopoiesis
- High risk of infection (granulocytopenia), and haemorrhage (thrombocytopenia)
- Metabolic complications
- Hyperuricemia, hyperphosphatemia, hyperkalemia – due to high cell turnover in malignant cells
- Tumour lysis syndrome with ARF – due to urate crystals depositing in tubules (during chemo)
- Allopurinol started and urine is alkalised prior to chemo to prevent this
- Hyperleukocytosis and leukostasis syndrome
- High blast count increases blood viscosity
- Leukostasis syndrome (blasts >50,000/Μl) – altered mental status, respiratory failure, CHF
- MC in AML
- Leukapheresis (removal of WBCs) used to reduce blast count
Diagnosis
- CBC and blood smear
- BM aspirate/trephine biopsy
- Cytochemical stains
- MPO – positive in AML
- Sudan black B – positive in AML
- PAS – positive in some ALL
- Flow cytometry
- Cytogenetics
Complications of therapy
- Chemo with cytotoxic agents causes BM aplasis which leads to cytopenias
- Infections – E.coli, K.pneumoniae, Pseudomonas, S.aureus. Fungal infections. Viral (HSV, VZV, CMV)
- Hemorrhage – due to thrombocytopenia. Prophylactic platelet transfusion given
- Other SEs – N, V, alopecia, infertility
- Cytosine arabinoside – cerebellar dysfunction
- Anthracyclines (daunorubicin/doxorubicin) – cardiomyopathy
- Therapy-related AML
ACUTE MYELOID LEUKEMIA
Pathophysiology and Classification
- Very heterogeneous – can show differentiation along any lineages
- De novo AML – in patients with no previous hx of hematologic disease
- Patients trend to be younger, better survival
- Secondary AML – in pts with preceding hematologic disease or who have receive chemo for another malignancy
- In older patients, poorer prognosis
- FAB classification – based on morphology and cytochemical stains
- Doesn’t include cytogenetic abnormalities, presence of dysplastic features or other prognostic factors
- Criterion for AML dx is that ≥30% cells in BM/blood must be myeloblasts (in WHO it is lowered to 20%)
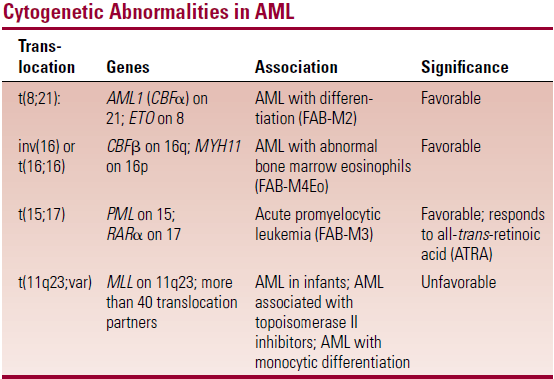
- M2 t(8:21), M3 t(15:17), M4 t(16:16) are the most common categories
- M3 shows distinct auer rods and M4 gum infiltration
Epidemiology
- MC in older adults
- Predisposing factors – Downs syndrome, Fanconi, ataxia-telangiectasia. Familial predisposition. Ionizing radiation. Benzene
Clinical features
- Resembles the ALL symptoms of BM infiltration and suppression of normal haematopoiesis
- Fever, mild splenomegaly
- Tissue involvement (MC in AML than ALL)
- Skin involvement – presents as violaceus non-tender plaques/nodules
- Gum involvement – bleeding
- Hyperleukocytosis with leukostasis – MC in AML than ALL
- Metabolic – hyperuricemia, hyperphosphatemia, tumour lysis syndrome with ARF
- Hypoglycemia – in patients with high blast counts (due to consumption of glucose by blasts)
- DIC – MC in AML
Diagnosis
- Anaemia and thrombocytopenia
- Blood smear
- Variable WCC – mostly increased, but can also be decreased
- Blasts – larger and more variable than those in ALL
- have more irregular nuclei
- Can be impossible to distinguish from lymphoblasts
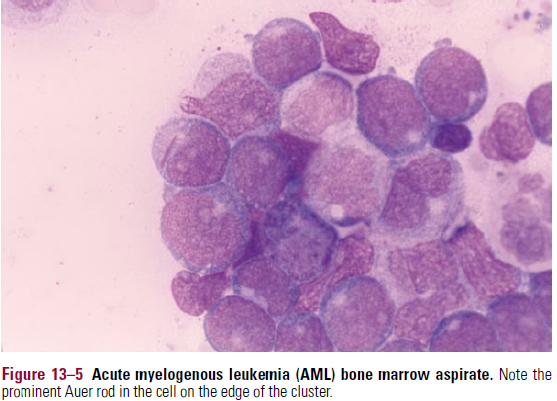
- Auer rods – absolutely confirms myeloid lineage (see BM pic)
- Bone marrow – hypercellular, with predominance of blasts/other immature cells
 Stains – see table
Stains – see table
- Immunophenotype – flowcytometry most useful in distinguishing between AML and ALL
- CD13, 15, 33 – Myeloid lineage-associated markers
- CD7 – in M0, M1
- Expression of HLA-DR – except on APL
- CD19 – suggests t(8;21) translocation – ALL
Cytogenetics
- Critical in diagnosis
- M2 t(8:21), M3 t(15:17), M4 t(16:16)
 Differential diagnosis
Differential diagnosis
- Reactive leukocytosis – has more mature cells than blasts
- ALL
- Myeloproliferative disorders (e.g. CML)
- Have rarity of blasts in blood and BM
- Myelodysplasia
- <20% blasts = Myelodysplasia
- >20% blasts = AML
Treatment
- Divided into remission induction and postinduction phases
- Remission induction
- Anthracycline + ara-C
- 3+7 regime – 3 daily doses of daunorubicin + 7 daily doses of ara-C
- Postinduction therapy
- Chemotherapy – high dose ara-C
- BMT – most effective to decrease relapse (may be contraindicated in older patients due to transplant-related mortality)
Prognosis
- Favourable – under 60s, t(8;21), (15;17). (16;16)
